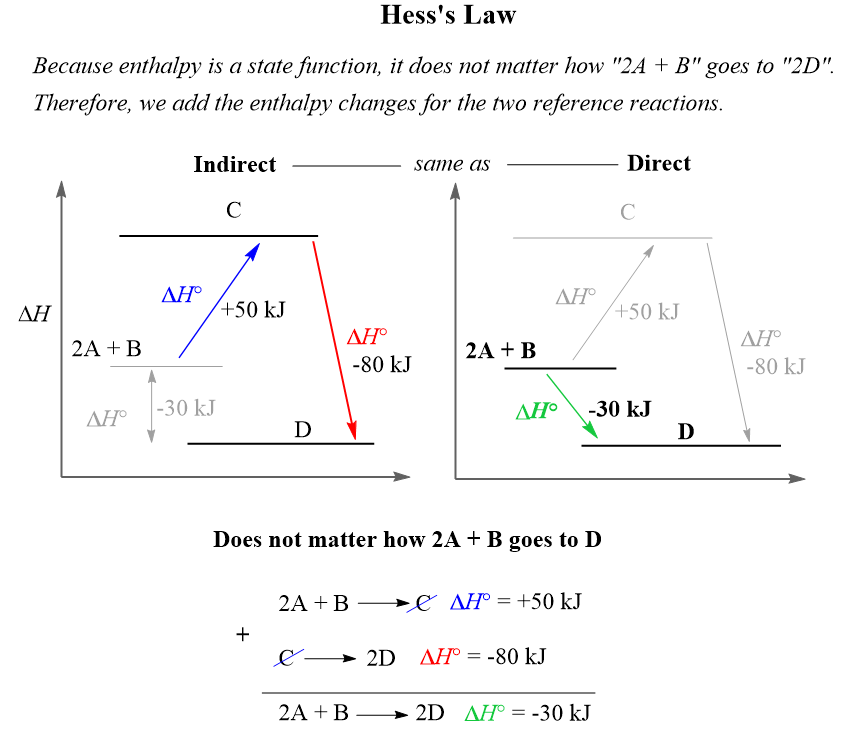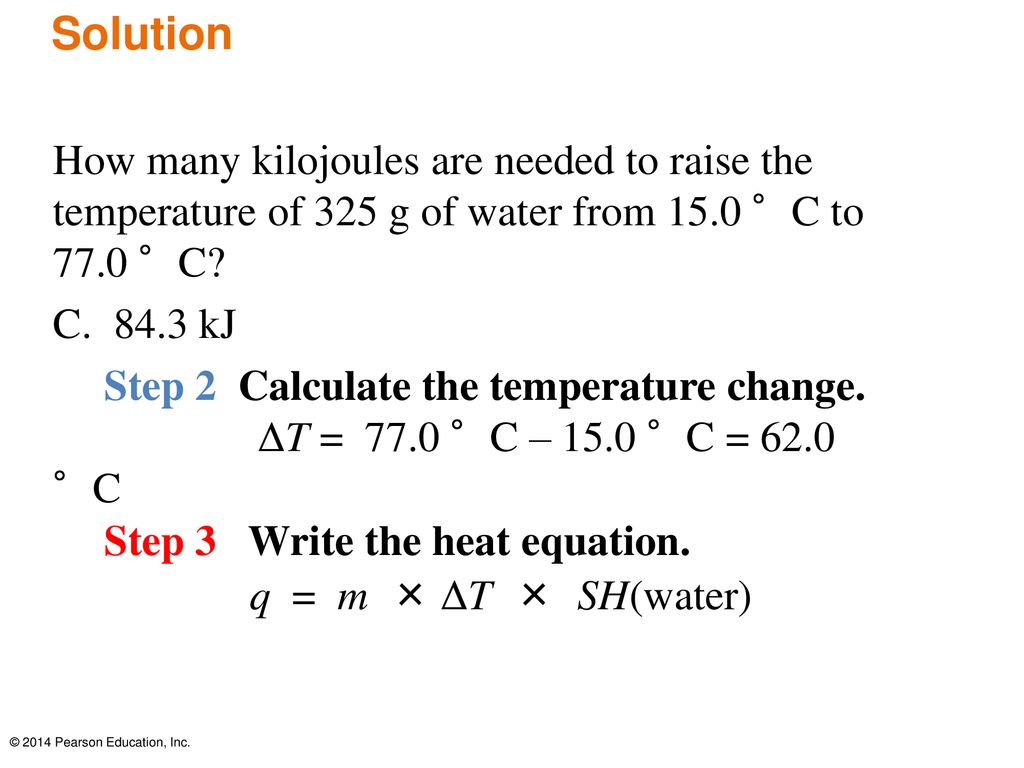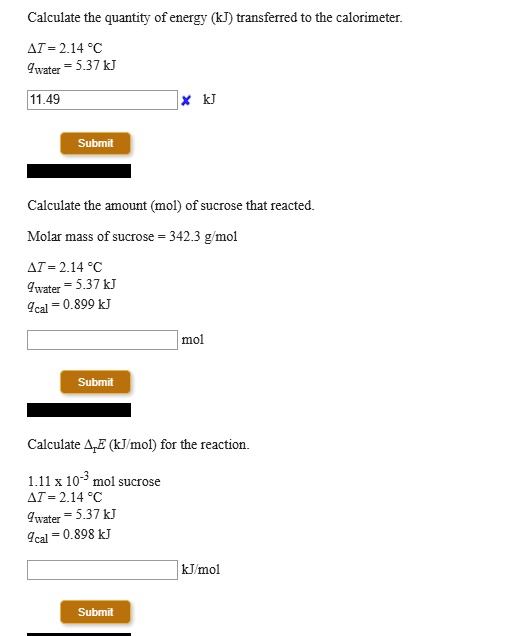
SOLVED: Calculate the quantity of energy (kJ) transferred to the calorimeter 1I=214"C Juzter 5337k 11.49 Submit Calculate the amount (mol) of sucrose that reacted Molar mass of sucrose 342.3 g mol 1T=214"C

The specific heat of the PCM is 2.3 KJ/kg/°C, the density of the PCM is... | Download Scientific Diagram

Calculate the number of KJ of heat necessary to raise the temperature of 60.0g of aluminium from.... - YouTube
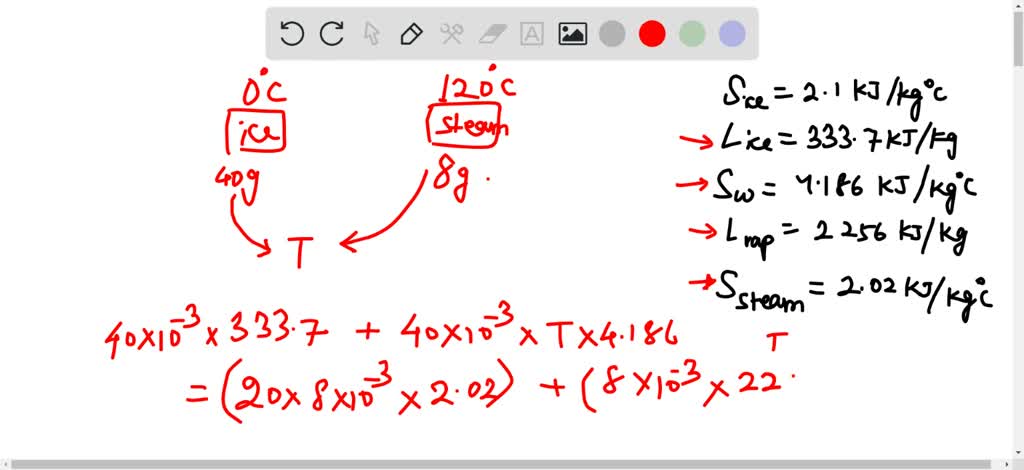
SOLVED: The specific heat of ice is 2.10 kJ/kg C, the heat of fusion for ice at 0 C js 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg C, the heat

The heat of combustion of C(graphite) is - 393.5kJ mol^-1 . The heat of formation of CO2 from graphite is kJ mol^-1 .
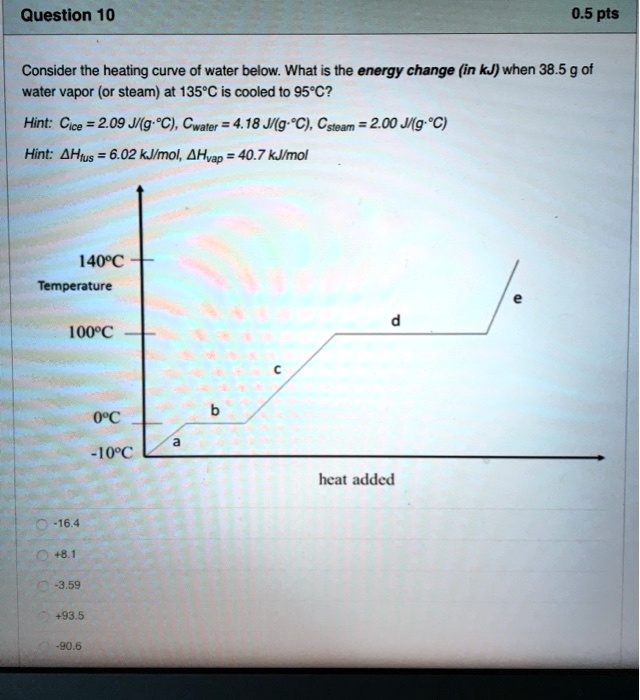
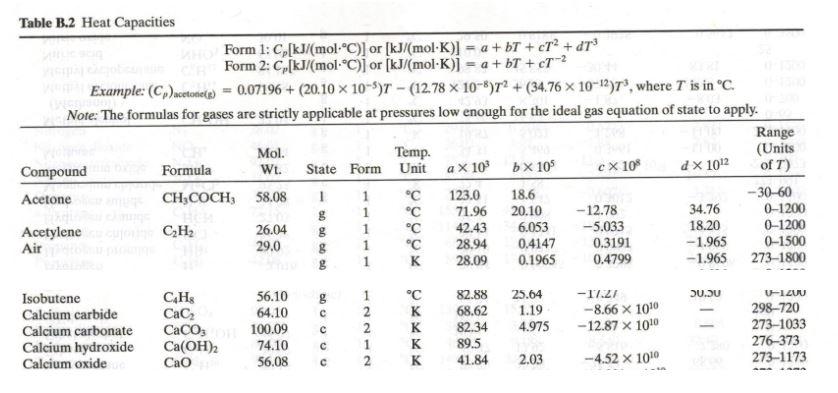
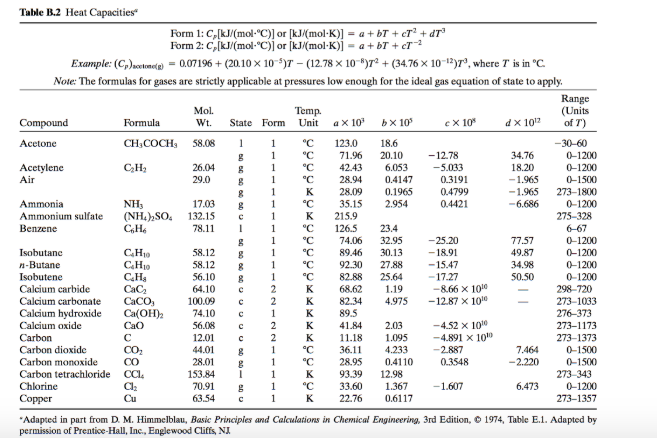
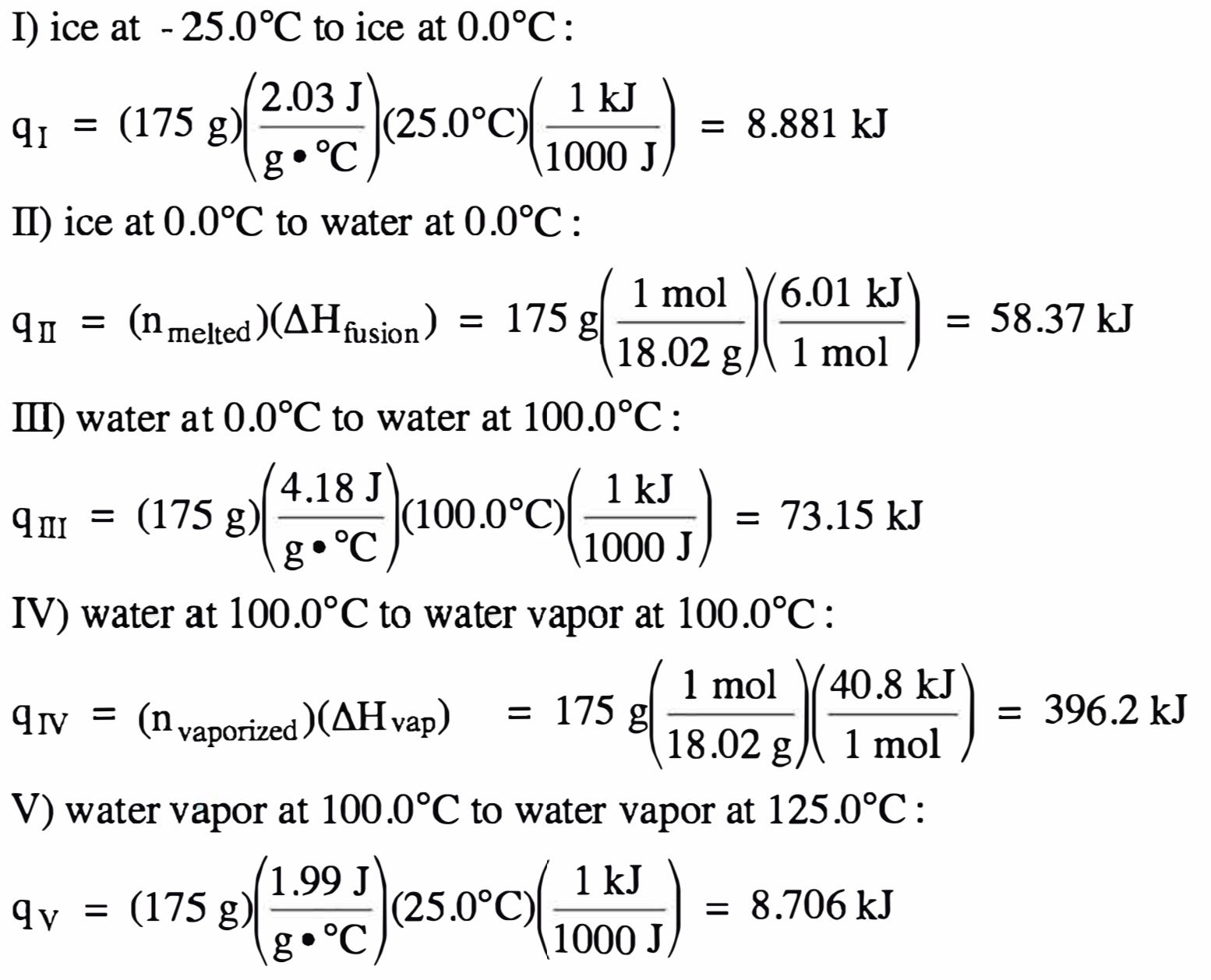


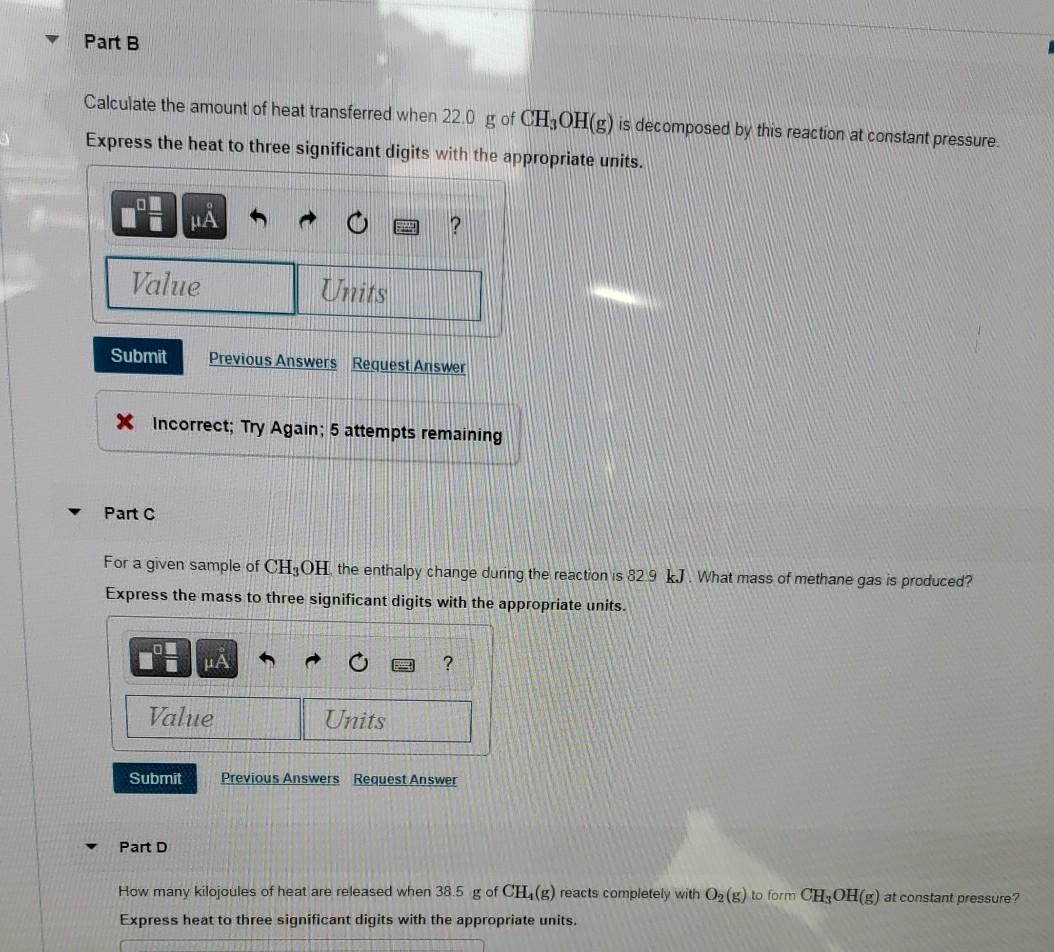
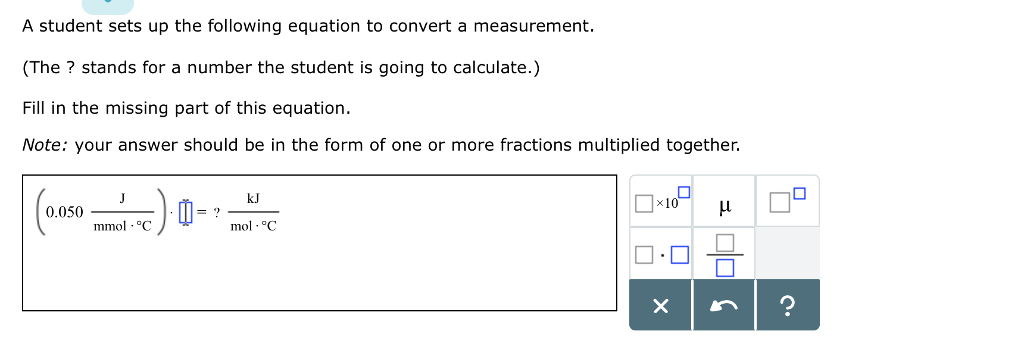


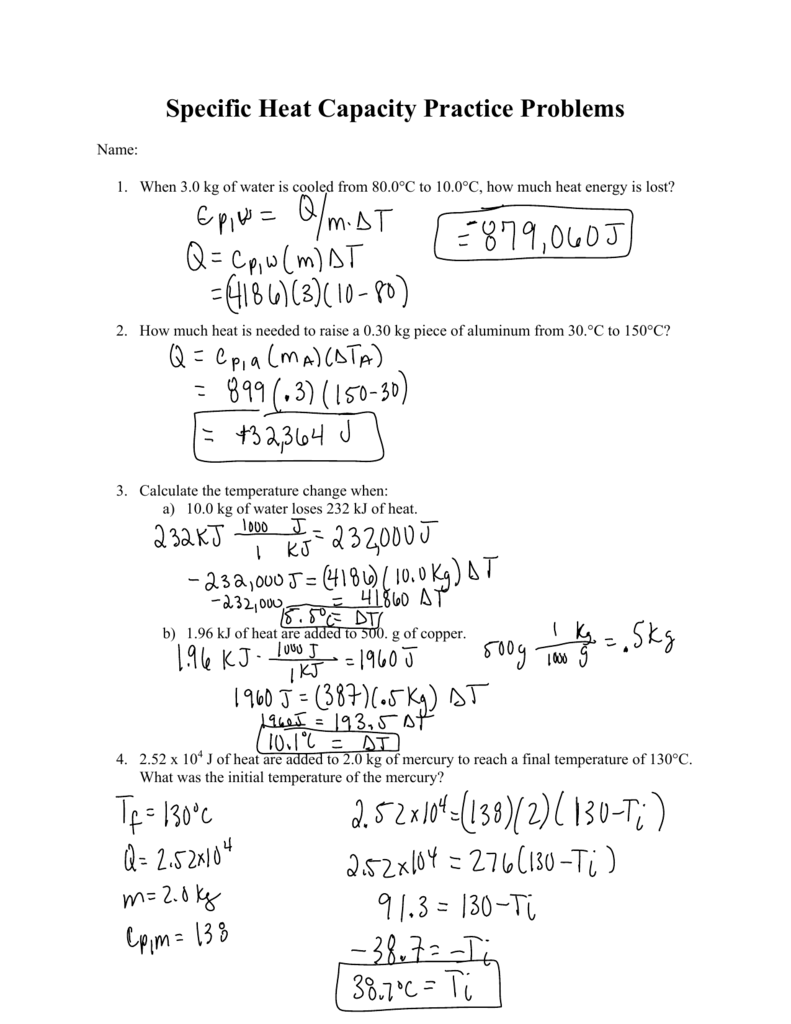
![Solved Table B.2 Heat Capacities Form 1: C1kJ (mol·°C)] or | Chegg.com Solved Table B.2 Heat Capacities Form 1: C1kJ (mol·°C)] or | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2Fd1e%2Fd1ec8c67-abd1-4bf2-88d0-ce524e9ef2b2%2FphpIIt2ym.png)